Abstract
Introduction: Camidanlumab tesirine (Cami), an antibody-drug conjugate targeting CD25, has shown single-agent anti-tumor activity and manageable toxicity in a phase 2 trial in relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL; Carlo-Stella, et al. 2022). In the phase 1 cHL trial, biopsy tumor cell CD25 histoscores (H-scores) were significantly higher in responders vs nonresponders and low baseline soluble CD25 (sCD25) was possibly related to response (Hamadani, et al. 2021). Separately, circulating CCL17 has been correlated with response to therapies for cHL (Plattel, et al. 2016).
Objective: To assess CD25, sCD25, and CCL17 as predictive biomarkers of clinical response to Cami in patients (pts) with R/R cHL.
Methods: This analysis was based on the open-label, multicenter, phase 2 study in pts with R/R cHL after ≥3 prior lines of therapy (or ≥2 in pts ineligible for SCT; NCT04052997). CD25 expression was assessed by immunohistochemistry (IHC) and H-score in all archival tumor biopsies and a subset of recent biopsies (archival biopsies taken after the last systemic anticancer treatment, including SCT if applicable); serum sCD25 and CCL17 were assessed by qualified immunoassays prior to Cami infusion on day 1 of each cycle. Cami may interfere with the sCD25 assay, underestimating post-baseline (BL) sCD25. CD25 IHC, BL sCD25 and CCL17, and changes in sCD25 and CCL17 from BL were compared between responders and nonresponders. Receiver operating characteristic (ROC) analyses were performed to evaluate the predictive potential of these measures as response biomarkers.
Results: Assessments of CD25, sCD25, and CCL17 in pts with a best overall response (BOR) of complete or partial response (responders) and stable disease or PD (nonresponders) were performed for 111 of 117 pts treated with Cami (6 pts were not evaluable for BOR).
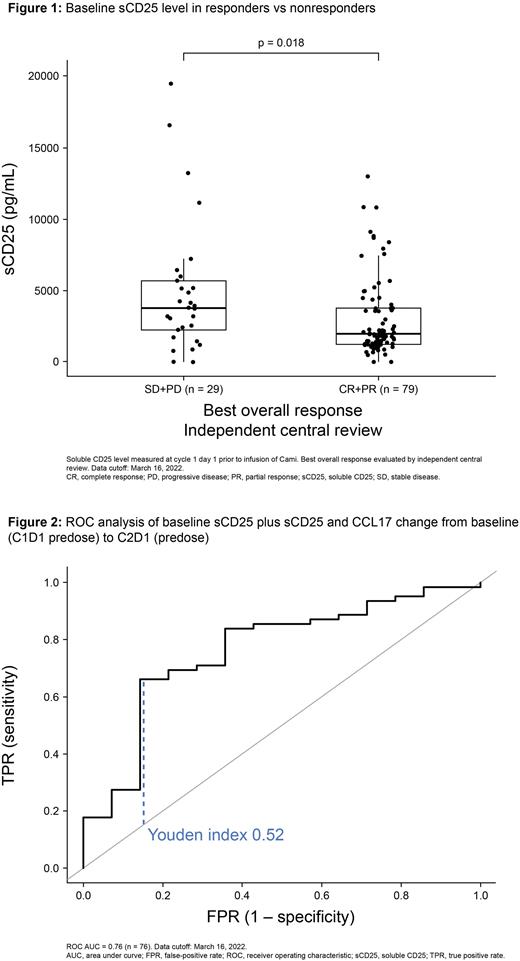
No significant difference in tumor cell CD25 H-score was observed between responders and nonresponders in all (n=99) or recent biopsies (n=23; p>0.05 for both), despite higher median levels in responders. BL sCD25 (n=108) was significantly higher in nonresponders than responders (p=0.018; Fig 1); though, overlapping values were observed in a substantial number of pts. There was also a significantly greater decrease in sCD25 in nonresponders vs responders from BL to C3D1 (n=79; median 0.53 vs 1.02; p=0.018). While there was no significant difference in BL CCL17 levels (n=106), there was a significantly greater decrease in BL CCL17 in responders vs nonresponders at C2D1 (n=97; median 0.50 vs 0.81; p<0.01 log10 change).
In ROC analyses in a subset of 96 pts with CD25 IHC and BL sCD25 results available, sCD25 alone showed a modest predictive potential (area under curve [AUC]=0.65) that was not improved with the addition of tumor cell CD25 H-score (AUC=0.67). In a subset of 76 pts with results available for sCD25 and CCL17 at BL and C2D1, ROC curves of BL sCD25, BL sCD25 plus BL CCL17, sCD25 change from BL, and CCL17 change from BL showed modest predictive potential (AUC<0.7 for all). A better AUC was found in a model including change from BL at C2D1 of sCD25 and CCL17 (AUC=0.74). This AUC was further increased in a model of BL sCD25 and CCL17 plus their change from BL at C2D1 and a model of BL sCD25 plus sCD25 and CCL17 change from BL at C2D1 (AUC=0.76 for both; Fig 2). The latter model had a higher Youden index.
Conclusions: Higher median tumor cell CD25 H-score was seen in responders vs nonresponders in all and recent biopsies but the differences did not reach significance. These data warrant further investigations as phase 1 data showed significantly higher tumor CD25 expression in responders (Hamadani, et al. 2021). Nonresponders had significantly higher BL sCD25; however, this parameter alone has insufficient predictive potential for Cami efficacy.
Predictive models including BL sCD25 plus sCD25 and CCL17 change from BL at C2D1 (± BL CCL17) showed the best ROC AUCs but need further refining to be clinically relevant. However, given the high overall and early responses in most pts treated with Cami, biomarkers predicting response at C2D1 are suboptimal unless reaching a higher sensitivity and specificity.
Investigation of sCD25 in the context of the Cami mode of action and exposure-response and the potential value of sCD25 and CCL17 changes as early indicators of Cami's effect and as biomarkers for disease monitoring and response are ongoing.
Funding: ADC Therapeutics SA; writing: CiTRUS Health Group.
Disclosures
Dyczkowski:ADC Therapeutics: Consultancy. Herrera:Caribou: Consultancy; Pfizer: Consultancy; Genmab: Consultancy; Adicet Bio: Consultancy; KiTE Pharma: Research Funding; Gilead: Research Funding; Karyopharm: Consultancy; Regeneron: Consultancy; Tubulis: Consultancy; Takeda: Consultancy; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Carlo-Stella:Sanofi: Other: Consultancy/Advisory, Research Funding; Roche: Other: Consultancy/Advisory, Research Funding; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding; Incyte: Honoraria; AstraZeneca: Honoraria; Janssen Oncology: Honoraria; Merck Sharp & Dohme: Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Karyopharm Therapeutics: Other: Consultancy/Advisory; Scenic Biotech: Other: Consultancy/Advisory. Zinzani:Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment. Toukam:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Cruz:ADC Therapeutics SA: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Havenith:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Genmab: Patents & Royalties: Patent with Genmab. Boni:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wuerthner:Scenic Biotech: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Pantano:Merck: Current equity holder in publicly-traded company; ADC Therapeutics SA: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Pembrolizumab: Ended employment in the past 24 months; Novartis: Current equity holder in publicly-traded company, Other: Spouse works at Novartis; Alcon: Current equity holder in publicly-traded company; Organon: Current equity holder in publicly-traded company.
OffLabel Disclosure:
Camidanlumab tesirine is an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.